Good data from NTI’s Rett Trial
Today, our biotech Investment Neurotech International (ASX: NTI) released additional data on its Phase I/II Rett Syndrome trial.
The good news is that there was improvement on the core symptoms of the paediatric neurological condition out to 20 weeks.
This was across multiple measures
These were some of the highlights:
- 100% of patients showed improvement in core symptoms like communication, mental alertness, and anxiety (vs 93% at 12 weeks)
- 57% of patients were rated as "very much/much improved" (up from 36% at 12 weeks)
- 24% improvement in gold-standard Rett syndrome behaviour questionnaire scores vs baseline
- Zero serious adverse events, no weight loss, and improved safety profile compared to only approved Rett treatment Daybue
On that last point, we think that collectively this data points to NTI’s potential as an alternative to Neuren’s treatment for Rett Syndrome, given the strong evidence that NTI’s treatment has established a strong safety profile across this trial and other trials.
This is what NTI’s treatment does to one measure of Rett Syndrome symptoms out to 20 weeks:
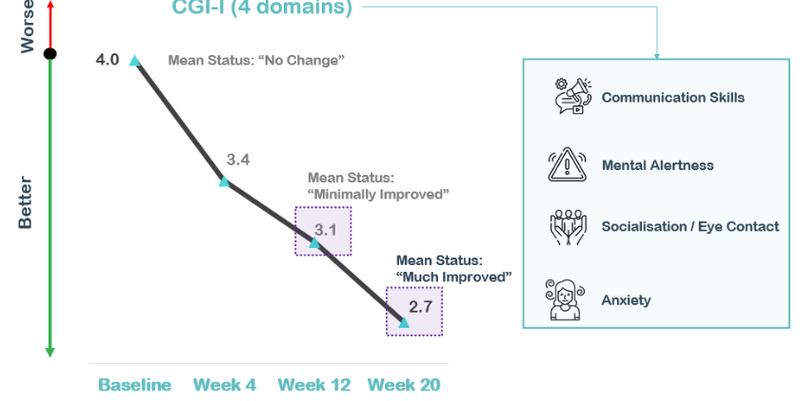
(Source)
By week 20, the mean status of those on NTI’s treatment is “much improved” which we take as an important win for the company.
As long term NTI Investors, what we’ve really enjoyed seeing across the company’s multiple successful clinical trials is the sustained nature of the benefits that the treatment provides to patients.
We saw this for example in NTI’s Phase II/III Autism Spectrum Disorder (ASD) trial which has continued to show increasing benefits out to 12 weeks.
Meanwhile, patients in the previous Phase I/II ASD trial have remained on the treatment out to 90 weeks.
We think this is what regulators look for in a treatment for long term conditions - symptoms get better over time AND a strong safety profile such that it can be taken for a long period.
Basically, a treatment that works and patients feel comfortable taking.
How does this news impact our NTI Investment Memo?
We see this as further positive progress across our number one Objective for NTI:
Objective #1: Rett Syndrome clinical trial
NTI is currently undertaking a Phase I/II trial that will test the safety and efficacy of NTI’s treatment for a rare genetic neurological and developmental disorder called Rett Syndrome.
This is the same disease that Neuren Pharmaceuticals commercialised a treatment for which helped take Neuren to a ~$1.4BN market cap.
NTI’s trial is aiming to improve on Neuren’s results across safety and efficacy, after dosing the first patient in this trial in August 2023.
Source: NTI Investment Memo 18 September 2023
We note that NTI’s Executive Director, Dr Tom Duthy also said that after today’s results, NTI is putting together a clinical trial design for a Phase III study on Rett Syndrome.
If NTI is able to prove out its treatment in a bigger cohort study that would be quite the achievement…
What’s next for NTI?
- FDA response on Orphan Drug Designation expected in ~3 months
- Advancing NTI164 through clinical pipeline across multiple rare neurological disorder indications (including Cerebral Palsy)
For a full rundown of what we want to see next from NTI, read our latest note:

NTI’s trial of Autism treatment in children shows further patient improvement after 12 weeks






